Some years ago I learned that the soaps and lotions and shampoos we moderns use can be nearly as harmful – over the long haul – as the cosmetics used by the ancient Egyptians or the Renaissance English and Europeans. I looked for safer alternatives. Finding them was quite a search. Many offered on the marketplace were just as bad or didn’t work or irritated my sensitive skin.
Some years ago I learned that the soaps and lotions and shampoos we moderns use can be nearly as harmful – over the long haul – as the cosmetics used by the ancient Egyptians or the Renaissance English and Europeans. I looked for safer alternatives. Finding them was quite a search. Many offered on the marketplace were just as bad or didn’t work or irritated my sensitive skin.
Eventually I found a handful of products that worked for me. (Discovering along the way that conventional products had been irritating my skin in a chronic, low level way that I thought was normal for me. It was not.)
But my hair was shorter then. As it’s grown to shoulder length, I’ve found myself wishing for some kind of conditioner. Not enough so as to take up another laborious search. More as a passing wish when I combed my hair.
I’m not really sure how I stumbled upon homemade lemon rinse. Something must have prompted me to do a little googling, but I no longer remember what it was. I bopped around a few websites, and what I learned made me decide to give it a try. Naturally I’m going to share my experience with you. 😀
First some basics.
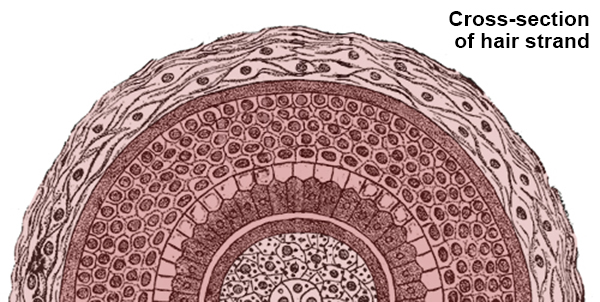
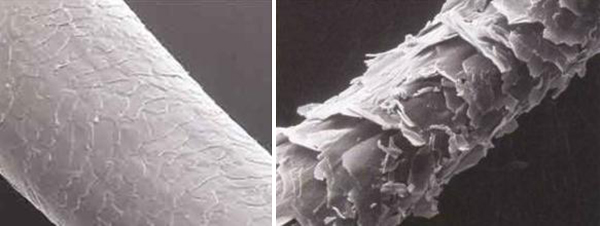
A strand of hair has layers, sort of like an onion (or an ogre, if that ogre is Shrek). At the core is a pith or marrow that is light and airy. It occupies about one-third the diameter of the strand. Around it are rod-like bundles of keratin. And on the outside is the cuticle, a layer of flat, thin cells that overlap one another like roof shingles.
Normal hair is somewhat acidic.
Substances that are acidic have a pH between 0 and 6.9. While those that are alkaline have a pH between 7.1 and 14. (A pH of 7 is neutral.) Human hair varies between 4.5 and 5.5.
This natural acidity of human hair prevents fungi and bacteria from growing on it. That’s critically important, obviously, but the acidity serves one other important function. When the hair strand has its proper acidity, the cells of its cuticle lie flat and tight, creating a smooth outer surface. When the hair is less acidic than it should be, the cuticle cells loosen and flap, creating a rough surface.
For this reason, a vinegar or lemon rinse serves as a beautiful conditioner.
Reading about it, I wasn’t sure I believed it. It seemed to simple. Too easy. But I decided to try it.
I purchased some ReaLemon® juice and mixed 2 tablespoons of it with 1-1/2 cups water, and poured the solution into a ketchup dispenser.
 (You need to dilute the lemon juice with water, because undiluted lemon juice is too acidic. You want a rinse that will put your hair right smack in the middle of its natural range.)
(You need to dilute the lemon juice with water, because undiluted lemon juice is too acidic. You want a rinse that will put your hair right smack in the middle of its natural range.)
When I next washed my hair, after I’d rinsed out the hairwash under the shower, I poured my homemade lemon rinse over my tresses, gently working it into the strands and into my scalp (which should also be mildly acidic).
I was astounded to notice that my hair did indeed feel slippery, just like with using a conventional conditioner, except without that super gooey, gunky feeling. My hair felt slippery, but still clean. Once I was out of the shower, dry, and in my robe, I took a comb to my hair. And was delighted to have the comb slide through the strands easily. Yay! Total success!
ETA: I did rinse the lemon rinse out of my hair after I’d worked it in. You don’t want to simply hop out of the shower leaving the lemon in. Let it do its work of making the hair strands properly acidic and then rinse the lemon away.
That experiment was 6 months ago, and I’ve continued to be very pleased with the results of my lemon rinse. It works. Simple as that.
I do have one caution, if you decide to make your own lemon rinse experiment.
I like to keep my rinse right in the shower with my hairwash and soap. I can do so when I use ReaLemon® as my source for lemon, because it has preservatives in it. But one time I ran out of ReaLemon® and couldn’t find it at the grocery store. I purchased the type of lemon juice that must be kept refrigerated. After 3 days of sitting in my bathroom, my rinse had a nasty coating of mold floating on the top, and I had to throw it away.
So, if you prefer to avoid the preservatives (and I do in the food I eat, but I’m willing to suffer them in my hair rinse for the sake of convenience), keep your lemon rinse in the fridge, pull it out for your hair washing, and put it back after. 😀
For more about safe and effective toiletries, see:
Hair Wash with Rhassoul Clay
Great Soap & Etcetera Quest
Facial Soap Eureka
For more info about alternative hair care, see:
Kanelstrand.com